Plymouth Meeting’s Inovio Pharmaceuticals Tests Zika Vaccine on First Volunteer
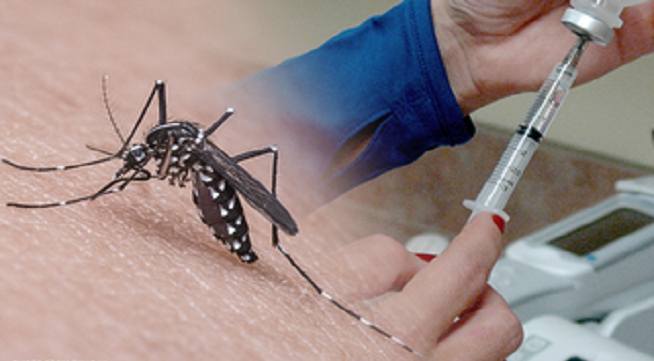
Plymouth Meeting’s Inovio Pharmaceuticals has injected the first volunteer with its experimental vaccine for the Zika virus, with results from the study expected later this year, writes Linda Loyd for the Philadelphia Inquirer.
Both the Food and Drug Administration and Health Canada’s Health Products and Food Branch have given the green light to early-stage tests in 40 healthy adults. The tests will be performed in Philadelphia, Miami, and Quebec City, to evaluate the safety, tolerability, and immunogenicity of the vaccine against the virus.
“The U.S. Centers for Disease Control estimates that there are 30 million to 40 million U.S. travelers to Zika-affected areas annually,” said J. Joseph Kim, Inovio president and CEO. “The resident population in the Americas at higher risk of Zika exposure has been estimated at nearly 300 million.”
Inovio has been developing the vaccine since last year with its South Korean partner, GeneOne Life Science, and in collaboration with the Wistar Institute in University City. The process uses DNA sequences from the Zika virus rather than the more traditional method of utilizing live bacteria from the virus itself.
Read more about the vaccine test in the Philadelphia Inquirer by clicking here.
Read MONTCO Today’s previous coverage of Inovio Pharmaceuticals and its Zika vaccine by clicking here.
Stay Connected, Stay Informed
Subscribe for great stories in your community!
"*" indicates required fields



![ForAll_Digital-Ad_Dan_1940x300[59]](https://montco.today/wp-content/uploads/sites/2/2022/06/ForAll_Digital-Ad_Dan_1940x30059.jpg)
























![95000-1023_ACJ_BannerAd[1]](https://montco.today/wp-content/uploads/sites/2/2023/03/95000-1023_ACJ_BannerAd1.jpg)














